Since the start of the pandemic, we’ve seen countless advancements in the medical field.

Over the course of the past few years, COVID-19 tests have become more rapid, vaccines have been developed, and we’ve even seen the addition of COVID-19 antiviral pills. But now, the FDA has approved a game-changing new device…one that can detect infections with only samples of a patient’s breath.
According to CBS News, the InspectIR COVID-19 Breathalyzer can yield results in just three minutes, however, it’s only available for use “by a qualified, trained operator under the supervision of a health care provider.” It’s designed to be used in hospitals, doctors’ offices, or mobile testing sites.
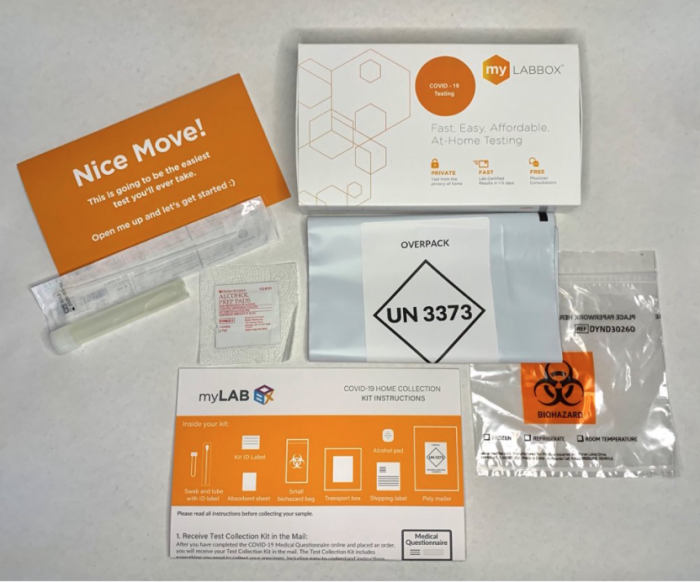
The device requires a piece of equipment that’s roughly the size of a piece of carry-on luggage. It was tested in a study of 2409 people (both with and without symptoms), and during those tests, the device “was able to spot 91.2% of cases — and yielded false positives in only 0.7% of results.” According to USAToday, the test correctly identified negative samples 99.3% of the time. The test is performed by exhaling into a tube — similar to blowing up a balloon.
Click Here to Learn More About the COVID-19 “Stealth Variant”
Join the AllEars.net Newsletter to stay on top of ALL the breaking Disney News! You'll also get access to AllEars tips, reviews, trivia, and MORE! Click here to Subscribe!
























Trending Now
Here are the park bag mistakes you might make when going to Disney World and...
If you're an introvert, should you head to GEO-82 or find another lounge in Disney...
Let's have a chat about an Oura Ring that Disney Adults just can’t seem to...
You need to check out these new Stanley Cup colors on Amazon!
There has been a BIG change in Magic Kingdom!
Going to Disney World alone isn't as scary as you'd think...
The resort's refurbishment lineup continues to grow.
It all starts on July 8th!
If you are flying home from Disney World with these souvenirs, be extra kind to...
Making park pass reservations as an Annual Passholder is changing BIG TIME this month!
This Disney Springs restaurant is MAJORLY underrated in my opinion, and I won't stand for...
Can't decide if you're going to spend money to see 'Moana 2'? Check out our...
We think this Polynesian Resort upgrade is totally worth it!
Get over to BoxLunch NOW!
We're expecting some HUGE Disney World news soon -- and if you're planning a visit...
Here are a couple of clues about Mickey's Very Merry Christmas Party 2025 at Disney...
As a Disney Pro, I'm always on the lookout for Disney essentials, and these are...
July is here! Can you believe we’re now onto the back half of the year?!...
We know, we know — it’s still summertime, but it’s always a good time to...
These EXCLUSIVE sodas are only available at Universal's newest park!